6 Minute read
University of Melbourne-based startup Navi has developed a location system to help clinicians insert life-saving central lines into premature and newborn patients. The team is preparing to begin clinical trials at Melbourne’s Royal Women’s Hospital and is on the road to US Food and Drug Administration regulatory clearance.
Key points
- Navi is developing a medical device that gives clinicians real-time feedback on the location of central venous catheters in children of all ages, including premature babies and newborns
- The Neonav improves patient safety and clinical workflow while reducing healthcare costs from misplaced or migrating lines
- Two out of five central line placement attempts into newborns currently result in a misplaced line, and up to a half may migrate to potentially unsafe locations within seven days
- Central lines deliver crucial nutrition and medication to premature babies and newborns
- Navi co-founders met at a University of Melbourne Master of Engineering graduate coursework subject and maintain strong ties with the University.
The outcome
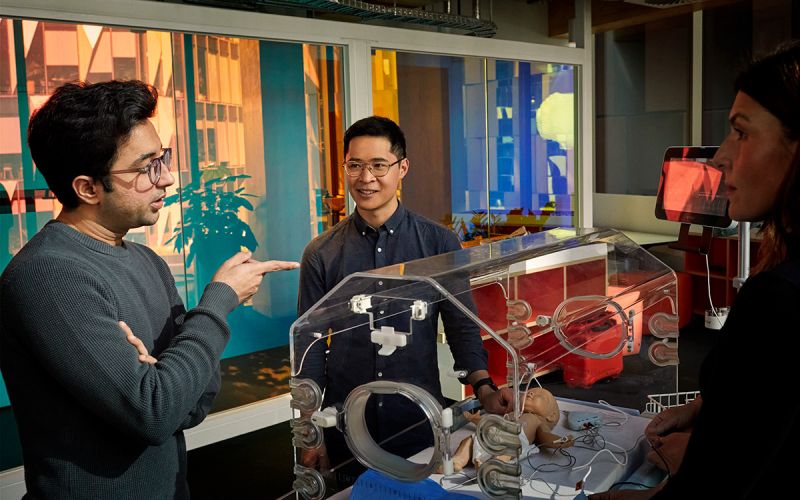
The Neonav electrocardiogram tip location system helps medical doctors insert central venous catheters to deliver drugs and nutrition to newborn patients.
Navi’s co-founders Mubin Yousuf and Shing Yue Sheung transformed a medical device invented for a university assignment into a startup company.
The Neonav alerts clinicians to the location of a central venous catheter in a premature or newborn patient’s body in real time.

This gives clinicians greater confidence in knowing exactly where the catheter is, and it also improves the clinical efficiency and improves patient outcomes as well. Shing Yue Sheung
Sheung completed both his undergraduate and masters degrees at the University of Melbourne.
The Neonav received Breakthrough Device designation from the US Food and Drug Administration (FDA) in 2022. The FDA gives this designation to devices that represent significant breakthroughs in effective treatment or aid in the diagnosis of life-threatening or debilitating conditions.
Convincing regulators that the Neonav is safe and effective is the next step needed to bring the device into hospitals. Clinical trials at the Royal Women’s Hospital are set to begin in late 2023 to early 2024.
“Every time we use the device on any patient, we'll be generating a data point that's saying that it works either the same as the standard of care or better than that. And a combination of those data points will then be submitted as part of a regulatory submission to the FDA, for example,” says Yousuf, a University of Melbourne Master of Engineering graduate.
Navi has received over $8 million in total funding through investment as well as grants to support the development of its device.
The need
“Premature babies need a lot of support,” says Yousuf.
“Because their digestive systems aren't really developed to the point that they can digest themselves, central lines can really be helpful in delivering vital nutrition and medication directly to their heart.”
But inserting a central venous catheter through a tiny blood vessel in a tiny body can be challenging – even for clinicians with a lot of practice.
“A clinician we met had over 30 years’ experience looking after patients, and despite best efforts, she along with her colleagues can still have difficulty with the catheter placement procedure,” Sheung says.
Two out of five catheter insertion attempts result in a misplaced line. Up to half may migrate within seven days to potentially unsafe locations.
And the neonatal intensive care unit (NICU) has few tools to help. Less than 5 per cent of FDA approved medical devices are approved for use in newborns. Clinicians make do with devices and equipment designed for larger patients.
The Neonav could also have a huge positive impact in developing countries, where more babies are born prematurely and at low birth weight.
Back home in Pakistan, for example, when I went to one of the public NICUs, I found that everyone was getting limited emergency support – because there were a lot of premature babies being born at the same time. It's a prevalent issue there. And a device like ours could really, really help that population.Mubin Yousuf
To improve the care of premature babies, NICU clinicians in developing and developed countries need a device to help insert and monitor the location of central lines in newborn patients.
The research
Currently, clinicians find out whether a central venous catheter has been misplaced by taking an X-ray after the procedure.
The Neonav tells clinicians where the catheter tip is in real time by using signals from the patient’s heartbeat.
The components of the Neonav system are:
- A screen that operates Navi’s proprietary software
- An electrocardiogram (ECG) acquisition unit
- A remote control
- A catheter connector.
The system uses differences in the ECG data captured from the patient's skin compared to ECG data from the catheter connector to tell the clinician whether the tip is misplaced too high or too low.
To monitor the catheter’s location for migration, the catheter connector can simply be reconnected to the Neonav system.
The Neonav improves patient safety and clinical workflow while reducing healthcare costs associated with misplaced or migrating lines.
Technology development history
Navi and the Neonav were born at a University of Melbourne Master of Engineering graduate coursework subject, BioDesign Innovation.
During the subject, graduate students learn how to create successful medical devices. Engineering students team up with business students as well as people from medicine and law backgrounds. The subject takes the teams from identifying clinical needs to concept development and business implementation.
“By the end of this subject, we thought, well, maybe this isn't just a subject anymore. What if we take this further and actually start a company?” Sheung says.
Since then, Navi has conducted several trials to develop and refine the Neonav and its software.

This current prototype is the culmination of all the studies, the research, the testing, and everything that we have learned up to this point. Mubin Yousuf
The Royal Women’s Hospital clinical trial will be the first time clinicians are using the Neonav to receive real-time feedback on the catheter tip’s position. If it goes well, Navi will expand it to include more sites and patients to continue building the case for regulatory clearance.
Navi has maintained its strong links with the University throughout its journey. Sheung and Yousuf participated in the University of Melbourne’s Velocity Program – a “startup school” for founders. The program taught them how to further develop their product, how to validate their ideas with customers and how to pitch to investors.
“The program went for a couple of months, and then it culminated in a demo day at the end. We got to present, get up on stage, get our name out there. It was really good for exposure as well,” Sheung says.
The startup works from the Telstra Creator Space and holds student internships through the University. The University of Melbourne is also a Navi co-investigator on several research and development grants.
Learn more about entrepreneurship at the University of Melbourne
Partners
- Navi
- The University of Melbourne
- The Royal Women’s Hospital
- Design + Industry
Funding support
- $2.4 million in a funding round concluded on 1 August 2023, including $700,000 through Breakthrough Victoria
- $2.3 million Cooperative Research Centres Projects grant on 30 June 2023, in collaboration with The University of Melbourne, the Royal Women’s Hospital and Design + Industry
- $1.2 million through MTPConnect
- $600,000 Accelerating Commercialisation Grant through the Australian Government Department of Industry, Innovation and Science
- $178,000 through the Victorian Government Medtech Manufacturing Capability Program.
Awards
- Device designated as a Breakthrough innovation by the US Food and Drug Administration
- Gold prize at UCSF-Stanford Pediatric Device Consortium’s Michael R. Harrison Innovation Symposium pitch competition.
Patents
- (US 20210259778A1) titled “Catheter Location Determination In Pediatric Patients”
People
- Shing Sheung (co-founder and Chief Operating Officer)
- Mubin Yousuf (co-founder and Chief Technology Officer)
First published on 26 October 2023.
Share this article
Keep reading
-
Explore more MedTech research
Creating transformative solutions for patient treatment and recovery. We bring together expertise from engineering, technology, medicine and science to develop tools to improve community health and individual patient outcomes.
-
Why partner with us
Partner with the University of Melbourne. Join a community where the world’s best minds help solve the biggest global challenges of our time.
-
Collaborate with us
Explore some of the many ways you can partner with us to help your organisation excel.
-
Investing in University startups
Find out about new ways to invest in University startups